PA12: Polyamide 12
- Short Name
- PA12
- Name
- Polyamide 12
- Group
- ETP - Engineering Thermoplastics
- General Properties
- Chemical Formula
- Structural Formula
-

Properties
- Glass Transition Temperature
- 40 to 50 °C
- Melting Temperature
- 170 to 180 °C
- Melting Enthalpy
- 95 J/g
- Decomposition Temperature
- 465 to 475 °C
- Young's Modulus
- 1400 MPa
- Coefficient of Linear Thermal Expansion
- 120 to 140 *10¯6/K
- Specific Heat Capacity
- 1.17 to 1.26 J/(g*K)
- Thermal Conductivity
- 0.22 to 0.24 W/(m*K)
- Density
- 1.01 to 1.04 g/cm³
- Morphology
- Semi-crystalline thermoplastic
- General properties
- High impact strength. Good chemical resistance. Very good stress cracking resistance. Good sliding-friction behavior
- Processing
- Extrusion
- Applications
- Mechanical and apparatus engineering (e.g., bearing and drive elements in humid environments requiring high stability). Automotive engineering. Electrical engineering. Packaging. Medical engineering
Internet Links
NETZSCH Measurements
- Instrument
- DSC 204 F1 Phoenix®
- Sample Mass
- 11.55 mg
- Isothermal Phase
- 3 min
- Heating/Colling Rates
- 10 K/min
- Crucible
- Al, pierced
- Atmosphere
- N2 (50 ml/min)
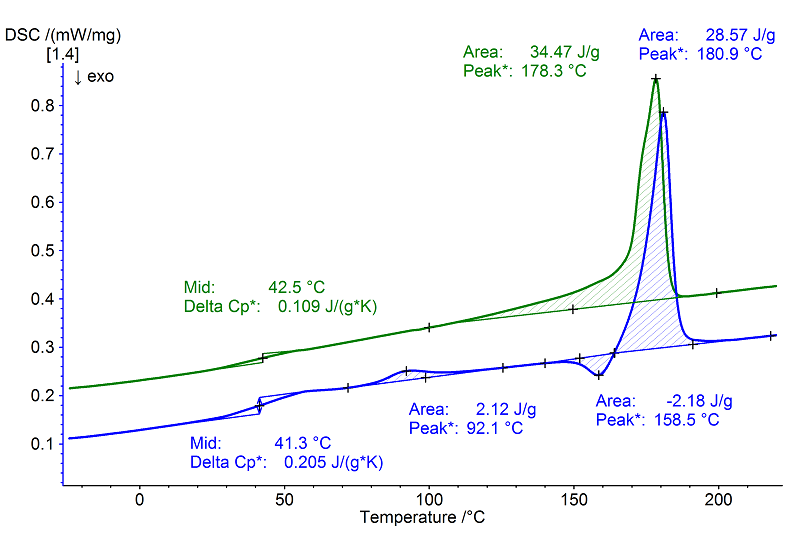
Evaluation
In this measurement, evaporation of water occurred at 94°C (peak temperature 1st heating, blue). The shift in the position of the glass transition in the 2nd heating (green, (Tg of 43°C – midpoint with a Δcp value of 0.11 J/(g·K)) after water evaporation was smaller than that on page 95. The cooling rate selected in the experiment (in this case 10 K/min) was lower than the cooling rates polymer granulates generally experience during production. Therefore, the amorphous content of the sample was lower in the 2nd heating. This thesis is confirmed by the relatively high step height (Δcp) of the glass transition in the 1st heating (blue) as well as the exothermal post-crystallization (peak temperature: 159°C, crystallization enthalpy: 2.2 J/g) that occurred immediately prior to melting.
The endothermal melting effect at 178°C (peak temperature, 2nd heating) exhibited a melting enthalpy of approx. 34 J/g.
The endothermal melting effect at 178°C (peak temperature, 2nd heating) exhibited a melting enthalpy of approx. 34 J/g.