PET: Polyethylene terephthalate
- Short Name
- PET
- Name
- Polyethylene terephthalate
- Group
- ETP - Engineering Thermoplastics
- General Properties
- Chemical Formula
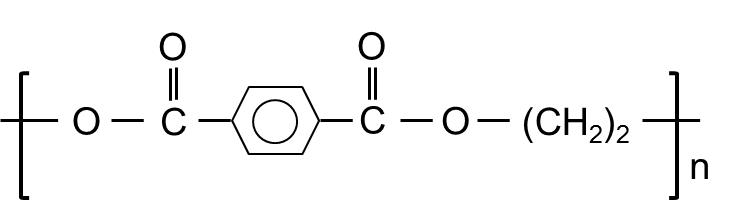
- Structural Formula
-
Properties
- Glass Transition Temperature
- 70 to 85 °C
- Melting Temperature
- 245 to 260 °C
- Melting Enthalpy
- 140 J/g
- Decomposition Temperature
- 425 to 445 °C
- Young's Modulus
- 2100 to 3100 MPa
- Coefficient of Linear Thermal Expansion
- 80 to 100 *10¯6/K
- Specific Heat Capacity
- 1.04 to 1.17 J/(g*K)
- Thermal Conductivity
- 0.24 W/(m*K)
- Density
- 1.33 to 1.45 g/cm³
- Morphology
- Semi-crystalline thermoplastic
- General properties
- High stability and stiffness. Good abrasion resistance. Good sliding properties. Resistant to diluted acids, aliphatic and aromatic hydrocarbons, oils, fats and alcohols. Tear and weather resistant. Good electrical insulating properties
- Processing
- Injection blow molding, stretch blow molding, injection molding
- Applications
- Fibers (polyesters), e.g., for sportswear. Packing (e.g., beverage bottles). Instrument and apparatus engineering. Medical engineering
Internet Links
NETZSCH Measurements
- Instrument
- DSC 204 F1 Phoenix®
- Sample Mass
- 8.43 mg
- Isothermal Phase
- 5 min
- Heating/Colling Rates
- 10 K/min
- Crucible
- Al, pierced
- Atmosphere
- N2 (50 ml/min)

Evaluation
Polyethylene terephthalate (PET) exemplifies how the ratio of amorphous and crystalline phases within a sample can be affected by different cooling rates. During production, the material undergoes very fast cooling, resulting in a high amorphous
content. This is evident in the 1st cooling (blue) from the large glass transition (step of Δcp of 0.34 J/(g.K)) and subsequent cold or post-crystallization at 137°C (peak temperature). Post-crystallization is generally associated with a volume change (shrinkage). At 251°C (1st heating, blue), all crystalline phases melt.
After a controlled cooling at 10 K/min, the amorphous content of the polymer was considerably lower than before. For this reason, the glass transition step height in the 2nd heating (green) was lowered and post-crystallization was almost completely eliminated. The melting temperature in the 2nd heating (peak temperature) occurred at 249°C. The difference between peak temperatures of the 1st and 2nd heatings is due to the better contact between the sample and crucible bottom after the first melting.
content. This is evident in the 1st cooling (blue) from the large glass transition (step of Δcp of 0.34 J/(g.K)) and subsequent cold or post-crystallization at 137°C (peak temperature). Post-crystallization is generally associated with a volume change (shrinkage). At 251°C (1st heating, blue), all crystalline phases melt.
After a controlled cooling at 10 K/min, the amorphous content of the polymer was considerably lower than before. For this reason, the glass transition step height in the 2nd heating (green) was lowered and post-crystallization was almost completely eliminated. The melting temperature in the 2nd heating (peak temperature) occurred at 249°C. The difference between peak temperatures of the 1st and 2nd heatings is due to the better contact between the sample and crucible bottom after the first melting.